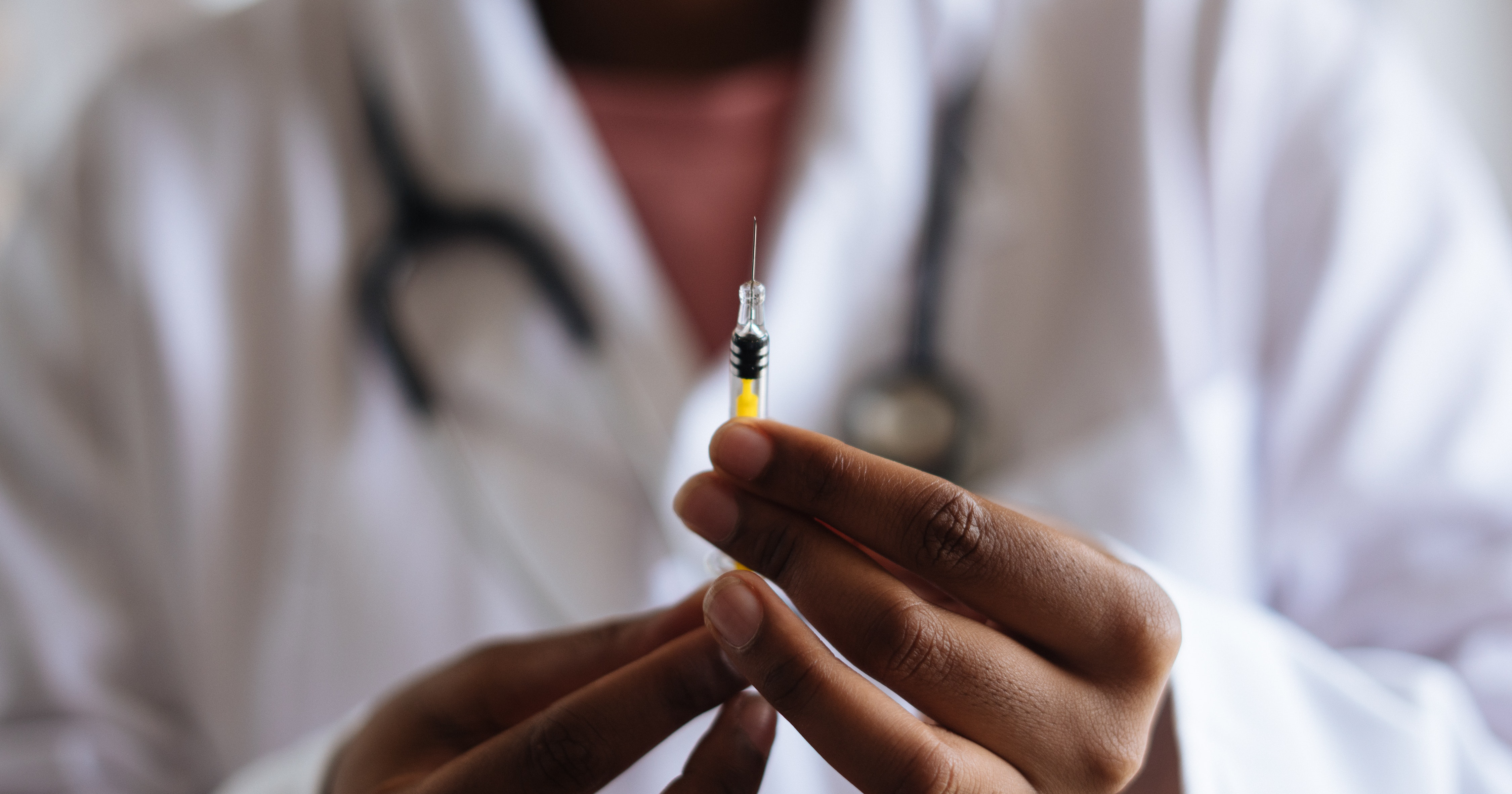
The US Food and Drug Administration (FDA) has approved a “game-changer” in the field of HIV prevention with an injectable PrEP treatment.
Instead of a daily PrEP pill taken orally, patients would have an injectable treatment (which will be known as Apretude) given every two months following two initial injections given one month apart.
Debra Birnkrant, director of the Division of Antivirals in the FDA’s Center for Drug Evaluation and Research, stated, “Today’s approval adds an important tool in the effort to end the HIV epidemic by providing the first option to prevent HIV that does not involve taking a daily pill.”
Birnkrant continued, “This injection, given every two months, will be critical to addressing the HIV epidemic in the US, including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option.”
As HIV Ireland explains, “PrEP, or pre-exposure prophylaxis, is a medication taken by HIV-negative people to reduce the chance of getting HIV from having sex without a condom and from sharing needles or equipment to inject or use drugs. The medication used for PrEP is a tablet which contains tenofovir and emtricitabine (sometimes known as Truvada).”
The information continues, “PrEP has been shown in many studies to be safe and highly effective at preventing HIV. PrEP stops HIV from establishing itself inside the body. When taken correctly PrEP has been found to be about 99% effective.”
In an interview with US TV show Good Morning America, Emergency Medical Physician Dr Darien Sutton shared, “This is a game-changer in the world of HIV prevention. Patients often have difficulty complying with any oral medication, so a bi-monthly injection can truly change the landscape in terms of HIV prevention. Having a bi-monthly treatment also serves as an opportunity to interact with a patient, share risk reduction sexual health education and complete necessary screenings.”
While the treatment reaching Ireland isn’t immediate, for current information on accessing PrEP, you can contact the MPOWER team at HIV Ireland here.
© 2021 GCN (Gay Community News). All rights reserved.
Support GCN
GCN is a free, vital resource for Ireland’s LGBTQ+ community since 1988.
GCN is a trading name of National LGBT Federation CLG, a registered charity - Charity Number: 20034580.
GCN relies on the generous support of the community and allies to sustain the crucial work that we do. Producing GCN is costly, and, in an industry which has been hugely impacted by rising costs, we need your support to help sustain and grow this vital resource.
Supporting GCN for as little as €1.99 per month will help us continue our work as Ireland’s free, independent LGBTQ+ media.
comments. Please sign in to comment.