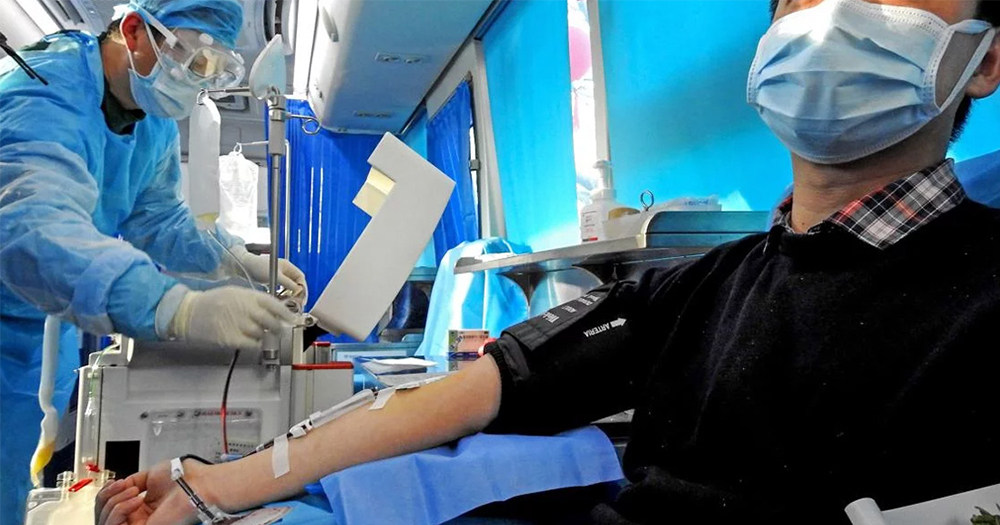
US health officials are required to turn away viable donors for an experimental treatment trial that could help patients diagnosed with COVID-19 due to restrictive policies on blood donation from GBT+ men.
The US Food and Drug Administration (FDA) has granted access to utilising blood donations from patients recovering from COVID-19 in order to treat the virus. Mount Sinai Health System in New York City, USA, are currently in the development stages of this potentially lifesaving treatment by collecting antibody-rich plasma from viable donors.
Despite the crucial nature of this treatment, GBT+ men can only donate after a year of abstinence from sex as a result of regulations introduced in 2015. In compliance with these restrictions, health officials are required to turn away viable donors.
According to NBC News, New York City resident Sabri Ben-Achour, aged 39, had contracted and recuperated from COVID-19. Based upon the ‘robust levels’ of antibodies in his system, Mount Sinai doctors wanted him to immediately donate his blood for use in an experimental infusion on critically ill COVID-19 patients.
However, Mount Sinai informed Ben-Achour moments before the scheduled appointment at the New York Blood Centre that he would not be able to donate because he was taking Truvada. He inquired into whether stopping the PrEP treatment for a month would allow him to donate, however, the health service officials were strict on the fact that he must abstain for the full 12 months.
Ben-Achour said, “I would be more than happy to go off of Truvada for four weeks. But obviously not for a year — that would be putting myself in danger.”
The blood bank which declined his donation insisted that they must follow federal and industry guidelines. A spokesperson told NBC News, “We are accredited by AABB (formerly the American Association of Blood Banks) and we follow their protocols, as well as FDA guidelines.”
President and CEO of LGBT+ advocacy group GLAAD, Sarah Kate Ellis, has called out the FDA policy as “irresponsible and illogical.” She said, “The FDA cannot let an outdated and discriminatory ban on blood donations from gay and bi men get in the way of potentially life-saving treatment for the country’s painful current health crisis.”
Ellis further stated, “Gay and bi men who have recovered from COVID-19 and want to donate plasma, or who want to help contribute to a nationwide shortage of blood, are banned from doing so as a result of the FDA. Continuing to enforce this antiquated policy is dangerous, irresponsible, and flies in the face of recommendations from medical experts.”
Numerous organisations and individuals across America have voiced their disagreement with the current system of blood donation especially in light of the ongoing COVID-19 pandemic.
A group of US senators called on the FDA to review its policy in a letter, which reads, “We must take every possible step to secure our nation’s blood supply in this critical time. In order to do so, we need to shift away from antiquated and stigmatising donation policies to ones that are scientifically sound, based on individual risk, and inclusive of all potential healthy blood donors.”
The FDA has also been heavily criticised by ACT UP NYC after they gave market exclusivity for the drug Remdesivir to Gilead for seven years, which was shown to be effective in treating patients with COVID-19.
© 2020 GCN (Gay Community News). All rights reserved.
Support GCN
GCN is a free, vital resource for Ireland’s LGBTQ+ community since 1988.
GCN is a trading name of National LGBT Federation CLG, a registered charity - Charity Number: 20034580.
GCN relies on the generous support of the community and allies to sustain the crucial work that we do. Producing GCN is costly, and, in an industry which has been hugely impacted by rising costs, we need your support to help sustain and grow this vital resource.
Supporting GCN for as little as €1.99 per month will help us continue our work as Ireland’s free, independent LGBTQ+ media.